
MY BACKGROUND
Throughout my life, I have fostered an immense passion and curiosity of the effects microorganisms exert on a host. This passion was further influenced when I started working as a certified, laboratory veterinary technician, and this prompted me to seek out more knowledge via Southern Illinois University (SIUC) in Carbondale, IL. At SIUC, I obtained a BS in biology in the biomedical sciences concentration along with a minor in chemistry. This was achieved while working in microbiological research lab that focused on molecular pathogenesis. As of 2018, I have been continuing graduate studies at Oklahoma State University (OSU) with the intent to gain a more profound understanding of the host immune response to chlamydial infection.
MY RESEARCH
As an undergraduate researcher and as a technician from 2013-2017, I was involved in lab work revolving around understanding the pathogenicity of Chlamydia trachomatis at a molecular level. I inactivated genes in vitro with the attempt to uncover their functions.
As a graduate student since 2017, I am now involved in lab work that focuses on immunological and histopathological effects of wild type and mutant strains of this pathogenic organism in a murine infection model.
All of the research that is being done is to advance the field of chlamydial genetic manipulation, to provide more of an understanding of the host response to infection, and to aid in the discovery of novel vaccine targets.
Research Interests:
Microbiology
Molecular pathogenesis
Host-Pathogen Interactions
Biotechnology
UTILIZATION OF MURINE INFECTION MODEL

Fall 2017-Current
This model is used to understand the histopathological and immunological effects of different Chlamydia trachomatis strains (mutant and wild type) that can be applied to what occurs in a human infection.
FLOW CYTOMETRY

Summer 2018-Current
During a genital chlamydial infection, there are a number of different immune cells that are recruited to the reproductive tract. Flow cytometry provides us with a quantitative analysis of each population of immune cells that are present in infected murine reproductive tracts.
This figure shows the relative neutrophil population from three, pooled murine reproductive tracts at day 7 post infection with a wild type strain of C. trachomatis.
CELL CULTURE AND MICROSCOPY

Fall 2013-Current
Fun Fact: Chlamydia spp. are obligate intracellular organisms. This means that to survive and grow, they have to be within a host cell.
With the lack of growth on agar, we rely on tissue cell culture to cultivate the organism.
This figure shows an immunofluorescent microscopy image of chlamydial inclusions stained with antibodies for improved visualization.
GENE INACTIVATION VIA TARGETRON
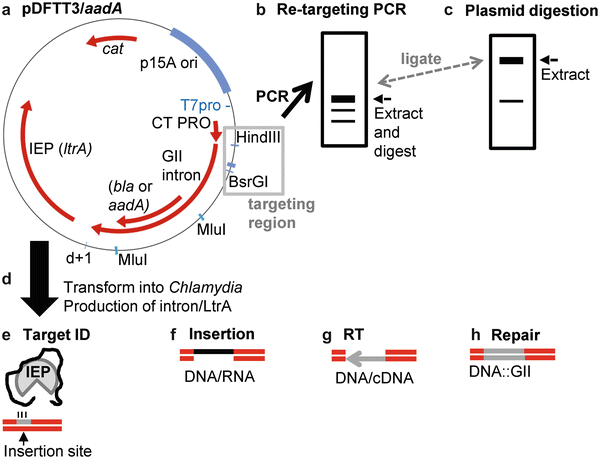
Fall 2013-Spring 2015
TargeTron is a gene inactivation system that involves the insertion of a mobile Group II intron into a gene of interest. This model was implemented and optimized for use in Chlamydia trachomatis with the hopes of determining the functions of hypothetical genes.
This figure shows a representation of the modification and insertion of a mobile Group II Intron into Chlamydia trachomatis using a shuttle vector (Key and Fisher, 2016) *See publications section*
